【Indications】
①Anemia caused by renal dysfunction, including dialysis and non-dialysis patients;
②Perioperative erythrocyte mobilization;
③Anemia caused by chemotherapy for non-myeloid malignancies. (To be added)
【R&D and Commercial Production of EPOSINO】
In 1991, technicians obtained the first batch of efficiently and stably expressed EPOengineered cell lines in China through genetic engineering technology, screening and various identifications;
In 1993, preclinical pilotscale production processes and preclinical animal trials were successfully completed in cooperation with relevant institutes, and various tests by the National Institute for the Control of Pharmaceutical and Biological Products were also passed;
In February 1994, the phase I clinical trial was approved by the Bureau of Drug Policy & Administration of Ministry of Health;
In January 1996, the phase II clinical trial was approved by the Bureau of Drug Policy & Administration of Ministry of Health;
In phase II clinical trial of EPOSINO, recombinant human erythropoietin originators (Epogen manufactured by Amgen in the USA, and Recormon manufactured by BoehrinMan in Germany) were used as reference substances in the pair therapy with EPOSINO. A total of 102 patients with anemia due to chronic renal failure were treated with EPOSINO. The efficacy was observed and compared: "The total effective rate of EPOSINO was 91.2%, and that of the original drug was 89.2%. The difference was not significant and the efficacy was equivalent" (data from the phase II clinical trial of EPOSINO).
In October 1996, the first new drug certificate issued by the Ministry of Health in China was obtained;
In October 1997, the first approval number for trial production in China was obtained;
In August 1998, the first approval number for formal production in China was obtained, and the new drug belonged to Class II biological products.
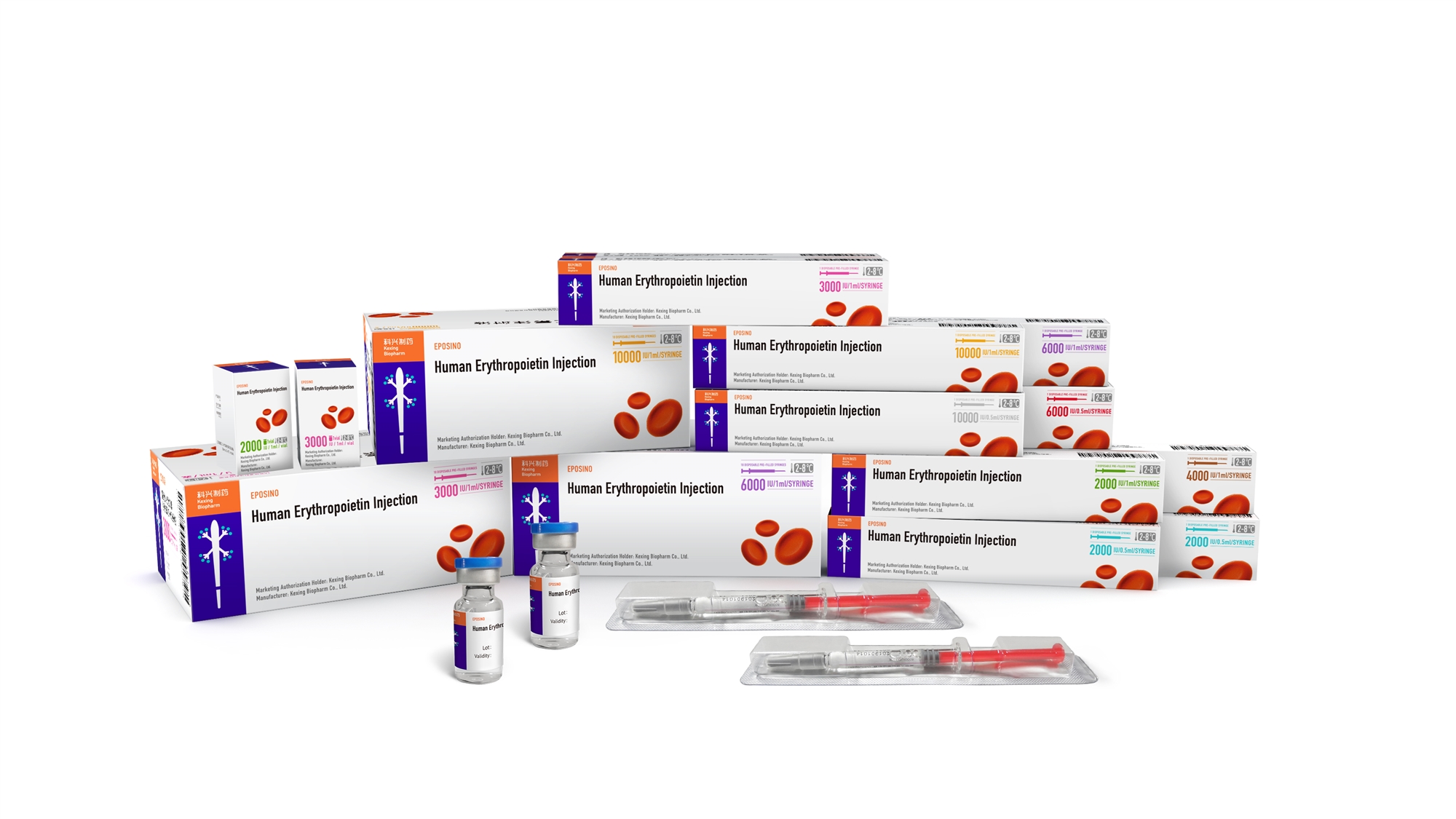
In 1999, the first prefilled syringe packaging was launch in China to facilitate clinical medication selection;
In 2006, clinical approval was obtained for the indication of perioperative erythrocyte mobilization;
In 2009, the albumin-free drug product was granted a patent for invention;
In 2011, the indication for perioperative erythrocyte mobilization was approved;
In 2013, supplementary approval was obtained for the albumin-free formulation;
In 2017, the clinical trial on the anemia indication of cancer chemotherapy was carried out;
In 2020, the marketing authorization application for the anemia indication of cancer chemotherapy was submitted;













 Scan Code Consultation
Scan Code Consultation